Impact of Auditory Integrative Training on Transforming Growth Factor-β1
and Its Effect on Behavioral and Social Emotions in Children with Autism
Spectrum Disorder - January 2018
Read more
clinical research on AIT
Contact Us
Complete
On-line AIT Checklist
Laila Al-Ayadhi,a
Abdulrahman Mohammed Alhowikan,b and
Dost Muhammad Halepotoa,*
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5968258/
Abstract
Objective
To
explore the impact of auditory integrative training (AIT) on the inflammatory
biomarker transforming growth factor (TGF)-β1 and to assess its
effect on social behavior in children with autism spectrum disorder (ASD).
Subjects and Methods
In this
cross-sectional study, 15 patients (14 males and 1 female) with ASD aged 312
years were recruited. All were screened for autism using the Diagnostic and
Statistical Manual of Mental Disorders (DSM-IV). Plasma levels of TGF-β1
were measured in all patients using a sandwich enzyme-linked immunoassay (ELISA)
immediately and 1 and 3 months after the AIT sessions. Pre- and post-AIT
behavioral scores were also calculated for each child using the Childhood Autism
Rating Scale (CARS), the Social Responsiveness Scale (SRS), and the Short
Sensory Profile (SSP). Data were analyzed using the Statistical Package for the
Social Sciences (SPSS 21.0 for Windows).
Results
Plasma
levels of TGF-β1 significantly increased to 85% immediately after AIT
(20.13 ± 12 ng/mL, p < 0.05), to 95% 1 month after AIT (21.2 ± 11 ng/mL,
p < 0.01), and to 105% 3 months after AIT (22.25 ± 16 ng/mL, p <
0.01) compared to before AIT (10.85 ± 8 ng/mL). Results also revealed that
behavioral rating scales (CARS, SRS, and SSP) improved in terms of disease
severity after AIT.
Conclusion
Increased plasma levels of TGF-β1 support the therapeutic effect of
AIT on TGF-β1 followed by improvement in social awareness, social
cognition, and social communication in children with ASD. Furthermore, TGF-β1
was associated with severity in all scores tested (CARS, SRS, and SSP); if
confirmed in studies with larger sample sizes, TGF-β1 may be
considered as a marker of ASD severity and to assess the efficacy of therapeutic
interventions.
Keywords:
Autism
spectrum disorder, Short Sensory Profile, Transforming growth factor-β1,
Childhood Autism Rating Scale, Social Responsiveness Scale
Significance of
the Study
This study
investigated the impact of auditory integrative training (AIT) on transforming
growth factor (TGF)-β1 and its effect on behavioral and social
emotions in children with autism spectrum disorder (ASD). The increased plasma
levels of TGF-β1 after AIT support the therapeutic effect of AIT on
TGF-β1 followed by improvement in social awareness, social cognition,
and social communication in ASD children. TGF-β1 may potentially
serve as a predictive biomarker of clinical symptoms of ASD and therapeutic
efficacy.
Introduction
Autism spectrum
disorder (ASD) is a neurodevelopmental disorder characterized by impairments of
social interactions, repetitive behavior, and sensory abnormalities [1]
with various levels of severity occurring before 3 years of age. ASD is an
important cause of childhood disability that imposes significant burden on the
parents and society [2].
Although the
exact cause of this disorder remains poorly understood, immunological factors
have been suggested to have a major role in its pathophysiology [3].
Several candidate molecules are emerging as promising biomarkers of autism and
may pave the way to better biological understanding of this condition. It is
also possible that the identification of reliable and robust biomarkers may
facilitate early diagnosis and personalized treatment, and improve outcome of
patients with autism [4].
Several ASD
screening and diagnostic procedures have been developed, including the Childhood
Autism Rating Scale (CARS) [5],
Social Responsiveness Scale (SRS) [6],
and Short Sensory Profile (SSP) [7].
According to several recent theories, sensory processing and integration
abnormalities may play important roles in impairments of perception, cognition,
and behavior in patients with autism. Among these sensory abnormalities,
distortion of auditory perception could contribute to many typical symptoms of
autism [8].
Impairment in sensory processing has been reported in 4288% of children with
autism; however, observational research examining the existence of sensory
processing dysfunction in autistic children is rare. Furthermore, little
attention has been given to research on the relationship between sensory
processing dysfunction and biomarkers that are measured in autistic patients [4].
Early
intervention has been shown to improve the prognosis of children with ASD [9],
but the most beneficial method of intervention remains unclear [10].
Tapping into the auditory strengths and preferences of children with ASD may
well lead to an improved option for early language intervention as audition
bears great significance in the acquisition of verbal speech, a lingering
challenge for about 30% of children with ASD [11].
Auditory
integrative training (AIT) was developed as a technique for improving abnormal
sound sensitivity in individuals with behavioral disorders including autism [12].
Berard [13]
suggested that the abnormal sensitivity or insensitivity to certain sound wave
frequencies, regardless of overall hearing ability, is associated with a range
of behavior and learning problems, and that AIT would bring about a
reeducation of the hearing process.
A number of
studies suggest that AIT plays a crucial role in social behavior and that it
could greatly improve language disorders, difficulties in social interactions,
typical behavior symptoms, and developmental levels [8,
14]. These studies report significant improvements in behavior and severity
of autism in terms of verbal and IQ performance 312 months after an
intervention. Furthermore, several studies have confirmed the effects of AIT on
social communication and interaction in ASD [8,
12]. Russo et al. [15]
also assessed the impact of auditory training on auditory function, and
identified biological changes, including brainstem response timing, pitch
tracking, and cortical response timing in children with ASD.
TGF-β1
is an anti-inflammatory cytokine that performs many cellular functions,
including the control of cell growth, cell proliferation, cell differentiation,
and apoptosis [16].
TGF-β1 has been found to play a crucial role in early central nervous
system (CNS) development [17]
and in the control of inflammatory and immune responses [18];
however, it can worsen brain inflammation when it is overexpressed in the brain
[19].
At the same time, it is believed that TGF-β1 protects the brain from
neuronal degeneration during CNS inflammation [20].
Several studies
have demonstrated altered TGF-β1 levels in the brain and serum of
autistic patients [21,
22]. Hashim et al. [23]
found that TGF-β1 levels were significantly decreased in the plasma
of ASD children in comparison to controls. Similar results were reported by
other studies in patients with ASD [22,
24].
Considering the
key role of TGF-β1 in brain development [17],
it is of great interest to study the role of TGF-β1 in the
pathophysiology of autism. Therefore, the aim of this study was to test the
possible effects of AIT on TGF-β1 and the association between plasma
TGF-β1 levels and the severity of social and cognitive dysfunction in
ASD children.
Methods
Participants
Subjects for this
study were recruited from the Autism Research and Treatment Centre at the King
Saud University, King Khalid University Hospital Riyadh, Kingdom of Saudi
Arabia. Fifteen ASD subjects, 1 girl and 14 boys (ranging in age from 3 to 12
years), were enrolled in the study. All subjects were screened and assessed
using the Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV. Scores
were calculated before and after intervention (immediately and 1 and 3 months
after AIT) for each child using CARS, SRS, and SSP. AIT was performed over 2
weeks, for a duration of 30 min, twice a day with a 3-h interval between
sessions.
Children with a
history of seizure were excluded from the study. Written consent was obtained
from the parents of each subject, according to the guidelines of the Ethics
Committee of the King Saud University King Khalid Hospital. During the study
period, children were not allowed to begin any new therapies or stop any current
therapies, including medications and supplements. Ethical approval was obtained
for the study by the Institutional Review Board of the College of Medicine, King
Saud University.
Childhood Autism
Rating Scale
The CARS score
was measured as a scale for autism severity. CARS assesses the child on a scale
from 1 to 4 in each of 15 dimensions or symptoms (including the ability to
relate to people, emotional response, imitation, body use, object use, listening
response, fear or nervousness, verbal communication, nonverbal communication,
activity level, level and reliability of intellectual response, adaptation to
changes, visual response, taste, smell and touch responses and general
impressions). A total score of at least 30 strongly suggests the presence of
autism. Children who score between 30 and 36 have mild-to-moderate autism while
those with scores between 37 and 60 have severe autism [25].
Social
Responsiveness Scale
The SRS is a
validated test of interpersonal behavior, communication, and stereotypical
traits in autism [6,
26]. It is used as a diagnostic tool, distinguishing clinically significant
ASD from varying levels of social impairment in other psychiatric disorders. It
consists of 5 subscales: (1) social awareness, (2) social cognition, (3) social
communication, (4) social motivation, and (5) autistic mannerisms. Total SRS raw
scores range from 0 to 195, corresponding to significant social impairment as
observed in individuals with ASD. A score of 76 or higher is considered severe
and is strongly associated with a clinical diagnosis of autistic disorder. A
score between 60 and 75 is in the mild-to-moderate range of social impairment [26].
The Short Sensory
Profile
The SSP is a
38-item questionnaire designed for children aged 314 years; it provides quick
information about the sensory processing skills of autistic children [4].
Each item on the SSP is measured on a 5-point Likert scale. Domain scores were
measured in the areas of tactile, taste/smell, and movement sensitivity, seeking
sensation, auditory filtering, low energy levels, and visual/auditory
sensitivity. Domain scores and overall sensory responses were categorized as
typical performance, probable difference from typical performance, or definite
difference from typical performance. Scores less than 142 indicate severe
performance (definite difference from typical performance), scores between 142
and 152 indicate mild-to-moderate performance (probable difference from typical
performance), and scores between 153 and 190 indicate typical performance. The
SSP has been used in many studies [7].
Auditory
Integration Training
AIT was conducted
according to a published protocol [13]
and previously used by our group [27].
Subjects were first examined by a medical doctor to ensure that no excessive wax
and/or fluid are present. The listener received 1820 listening sessions lasting
30 min, over a 10- to 20-day period in most cases, and had a 1- or 2-day break
after 5 days of listening. During the listening sessions, the child listened to
processed music. That is, the AIT sound amplifier attenuated low and high
frequencies at random from the compact disks and then sent this modified music
through headphones to the listener. The intensity level (volume) during the AIT
listening sessions did not exceed 80 dBA (low scale) and was set at much lower
intensities depending on the individual's comfort level. Overall, the music was
played at a moderately loud, but not uncomfortable, level. The 80-dBA level for
a total of 1 h per day is well below the Occupational Safety and Health Act
(OSHA) guidelines for nonhazardous noise levels. The OSHA Noise Standard permits
exposure to an average noise exposure of 85 dBA for 8 continuous hours.
Audiograms were obtained prior to, at the midpoint, and at the completion of the
AIT listening session. The first and the mid-point audiograms were used to set
filters on the AIT machines. These filters are used to dampen (40 dBA or more)
those frequencies which the person hears too acutely (i.e., peaks).
Blood Sample
Collection
After overnight
fasting, a 3-mL blood sample was collected from each child in test tubes
containing EDTA. Blood samples were immediately centrifuged at 3,000 rpm to
collect plasma, which was then stored in a freezer at −80°C until analysis. All
samples were assayed in duplicate and in a double-blind manner. Assay
reproducibility error ranged generally from 5 to 10%.
TGF-β1
concentrations were measured in the plasma of autistic subjects using a
commercially available sandwich ELISA kit (Cusabio Biotech Co. Ltd., Wuhan,
China). All biochemical analyses were performed in duplicate, and mean values
were reported. No significant cross-reactivity or interference was observed.
Statistical
Analysis
Data were
analyzed using the Statistical Package for the Social Sciences (SPSS 21.0 for
Windows; SPSS, Chicago, IL, USA). Results are expressed as means ± SD.
Significant changes in the parameters measured were assessed with
repeated-measure analysis of variance. Bonferroni multiple comparisons were also
used to assess significant differences. The Pearson correlation coefficient was
employed to determine correlations between TGF-β1 levels before and
after AIT. A value of p < 0.05 was considered significant.
Results
The changes in
TGF-β1 levels (means ± SD) and the scores of the 3 behavioral rating
scales (CARS, SRS, and SSP) before and immediately and 1 and 3 months after AIT
are listed in Table
Table1.1. Plasma levels of TGF-β1
significantly increased by 85% immediately after AIT (20.13 ± 12 ng/mL, p
< 0.05), by 95% 1 month after AIT (21.2 ± 11 ng/mL, p < 0.01), and by
105% 3 months after AIT (22.25 ± 16 ng/mL, p < 0.01) compared to before
AIT (10.85 ± 8). Scores of CARS, an indicator of autism severity, were decreased
by 17% 1 month after AIT (p < 0.05) compared to before AIT. SRS total
score significantly decreased (16%) and SSP total score increased (14%) 3 months
after AIT (p < 0.05). The significant difference (p = <0.01) in
SRS scores between 1 and 3 months after AIT also confirms the continuous
improvement in SRS behaviors with time and duration of AIT intervention. Results
revealed that these behavioral rating scales (CARS, SRS, and SSP) improved in
terms of disease severity after AIT sessions. Changes in the 3 behavioral rating
scales (SRS, CARS, and SSP) after AIT are shown in Figure
Figure11.
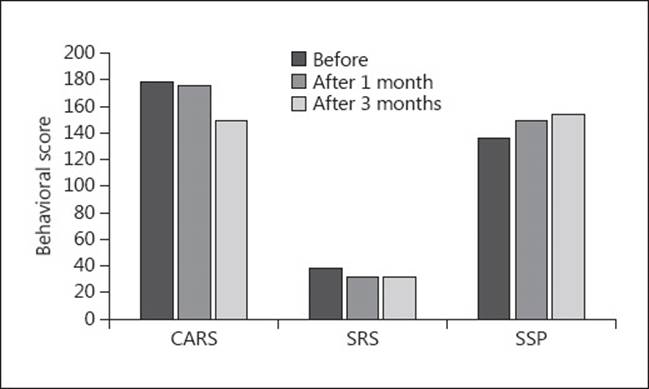
Fig. 1
Changes in the
Childhood Autism Rating Scale (CARS), Social Responsiveness Scale (SRS), and the
Short Sensory Profile (SSP) after auditory integrative training (AIT) in 3
different periods of time.
Table 1
Effect of
auditory integrative training (AIT) on transforming growth factor (TGF)-β1
and social behavioral scales in children with autism (n = 15)
|
Variable |
Before AIT |
Immediately after AIT |
1
month after AIT |
3
months after AIT |
Difference |
|
TGF-β1,
ng/mL |
10.85±8 |
20.13±12 |
21.2±11 |
22.25±16 |
1≠2*,
1≠3** |
|
CARS |
38.4±8 |
|
31.7±5 |
31.5±7 |
1≠3* |
|
SRS |
179±23 |
|
176±23 |
150±27 |
1≠4*,
3≠4** |
|
SSP |
136±22 |
|
150±26 |
155±24 |
1≠4* |
Means ± SD. CARS,
Childhood Autism Rating Scale; SRS, Social Responsiveness Scale; SSP, Short
Sensory Profile.
*p
< 0.05;
**p
< 0.01: before (1), immediately (2), and 1 month (3) and 3 months after AIT (4).
Pearson
correlation (r) values between TGF-β1 levels before and after
AIT are recorded in Table
Table2,2, showing strong and
significant correlations between TGF-β1 levels before AIT and
immediately and 1 and 3 months after AIT.
Table 2
Pearson's
correlation (r) between transforming growth factor (TGF)-β1
before and after AIT
|
TGF-β1 |
Before AIT |
Immediately after AIT |
1
month after AIT |
3
months after AIT |
|
Before AIT |
1 |
0.65N* |
0.74** |
0.591* |
|
Immediately after AIT |
0.65* |
1 |
0.50 |
0.414 |
|
1
month after AIT |
0.74** |
0.50 |
1 |
0.514 |
|
3
months after AIT |
0.59* |
0.41 |
0.51 |
1 |
*p
< 0.05;
**p
< 0.01.
Discussion
The findings of
this study show a significant increase in plasma levels of TGF-β1 and
improvement in some aspects of ASD behaviors. This was demonstrated by
significant changes in CARS, SRS, and SSP scores immediately and 1 and 3 months
after versus before AIT sessions. Higher levels of TGF-β1 and the
lower scores of CARS and SRS indicate less severity of autism. ASD is a complex
neurodevelopmental behavioral disorder with onset age prior to 3 years [1].
While there are no concrete biological markers for this disorder, immune
anomalies are frequently described among individuals with ASD [3].
Interaction
between speech and language systems is severely compromised in ASD. Sensory
dysfunction is a common finding in ASD, including tactile sensation, smell,
taste, visual, and auditory stimulation. Hypersensitivity to sensory stimuli is
considered a disturbing feature in autism, especially hypersensitivity to
auditory stimuli. This leads to communication difficulties which result in
social isolation and consequently in difficulties in rehabilitation and learning
[1].
AIT involves listening to music that has been computer modified to remove
frequencies to which an individual demonstrates hypersensitivities and to reduce
the predictability of auditory patterns. This treatment has been proposed to
improve abnormal sound sensitivity in individuals with behavioral disorders,
including ASD [13].
There is controversy in the literature regarding the effectiveness of AIT in
reducing the auditory hypersensitivity. A Cochrane review was conducted with the
objective to determine the effectiveness of AIT or other sound therapy methods
in individuals with ASD [28].
Three out of 6 trials reported improvements after 3 months of AIT using the
Aberrant Behavior Checklist (ABC) [29].
Results of the study conducted by our group [27]
also supported previous studies suggesting that AIT improved behavior of ASD
individuals [29,
30].
Our findings lead
us to suggest that increased levels of TGF-β1 following AIT in
children with ASD may be implicated in the pathophysiology of autism although
the result does not necessarily indicate causation. Furthermore, it is also of
interest to measure plasma levels of TGF-β1 in children without
autism after AIT in order to determine the role of TGF-β1 as a
serological marker for children with ASD.
It is possible
that high TGF-β1 levels may result in immune regulation after AIT and
thus possibly improve symptoms and behaviors associated with ASD. Furthermore,
peripheral immune markers may reflect biological factors that could affect
behavior in ASD children; however, further work is necessary to study the
precise role of TGF-β1 and how AIT is specifically linked to core
autism. As a major role of TGF-β1 is to control inflammation [18],
the positive correlations observed between TGF-β1 levels and AIT may
suggest that there is decreased inflammation in children who exhibit improved
behavioral scores. Further investigation is warranted on the use of TGF-β1
as a serological marker in children who have recently been diagnosed with ASD,
as well as its use as a biological marker to monitor potential efficacies of
therapies that target behavioral outcome.
Several
behavioral studies [8,
12,
14] have demonstrated the effects AIT on deficits in social communication
and interaction in ASD and significant improvements in behavior and severity in
autistic patients, but the effects of AIT on biochemical markers have not been
studied in ASD. Depino et al. [31]
described a central role of TGF-β1 in the programming and modulation
of social interaction and repetitive and depression-related behavior. They also
suggested a role for TGF-β1 and early-life neuroinflammation in the
development of behavioral alterations observed in ASD patients. These reports
suggest that immune system aberrations may lead to abnormal immune responses,
autoimmunity, or adverse neuroimmune interactions during brain development.
Given the key
role of TGF-β1 in brain development and inflammation, serum levels of
TGF-β1 were reported to be significantly lower in autistics than in
age- and gender-matched controls [21,
23]. The reduced TGF-β1 levels may lead to inappropriate
regulation of immune responses as well as the development of neuroinflammation
in ASD. However, the mechanisms underlying these processes have not been
elucidated.
Our TGF-β1
levels found before AIT intervention are similar to those reported earlier [21,
23] in typically developing children with autism, which significantly
increased up to 105% 3 months after AIT with improvement in social behavioral
functions. Taken together, these findings suggest that TGF-β1 may
play a role in the pathophysiology of ASD, although further work is needed to
confirm these reports. It is further recommended to measure plasma levels of
proinflammatory cytokines, such as IL-6 and TNF, that have actions opposite of
TGF-β1 to ascertain whether AIT is associated with changes in TNF as
well as IL-6, and thus the proinflammatory milieu is changed to an
anti-inflammatory status in ASD patients following treatment.
One of the
potential limitations of the present study is the small sample size; this may
have resulted in specific effects of AIT on TGF-β1 being missed
because of lack of statistical power to detect significant changes between the
TGF-β1 and behavioral scores (CARS, SRS, and SSP). We measured plasma
TGF-β1 levels before and after AIT, which might not accurately
reflect levels in the cerebrospinal fluid or in brain regions, whereas cytokines
readily cross the blood-brain barrier, suggesting that plasma levels should
correlate well with cerebrospinal fluid levels [32].
However, a disrupted blood-brain barrier has been demonstrated in autism [33].
The data from the present study pave the way for a larger, more focused study on
a wider range of cytokines. A further potential limitation of the present study
is that the exact mechanism of action of AIT remains to be elucidated. Finally,
an additional potential limitation of the present study is the fact that the
duration of AIT used may not have been optimal.
These findings
suggest that AIT could clinically alleviate an ASD core symptom about social
reciprocity with enhancement of brain activity and functional coordination in
ASD children. The current results may have a significant influence on future
biological studies and clinical trials of AIT effects on children with ASD. We
proposed that exposure to AIT sessions would result in improved behavioral
evaluation scores and positively influence TGF-β1 levels in autistic
children. However, these data should be treated with caution until further
investigations are performed in a larger study cohort, to determine whether the
increase in TGF-β1 levels is a mere consequence of autism or has a
pathogenic role in the disease.
Conclusions
Our findings
provide evidence for altered TGF-β1 protein levels in subjects with
ASD, which may contribute to the early pathogenesis of ASD, serve as a valuable
biomarker, and could be even predictive for the ameliorative effects of the
treatment, as studied here. Furthermore, in children with ASD there were
correlations between TGF-β1 levels before and after AIT. This finding
suggests that inflammatory responses may be linked to AIT. Overall, the results
of this study support the therapeutic effect of AIT resulting in increased TGF-β1
plasma levels and improvements in clinical ASD severity scores (CARS, SRS, and
SSP).
Future studies in
larger sample sizes including TGF-β1 determinations in normally
developing children (controls) are strongly recommended to assess the exact
beneficial effect of AIT and to ensure the greatest level of validity and
reliability.
Disclosure
Statement
The authors have
no conflict of interest.
Acknowledgments
We are grateful
to the Autism Research and Treatment Centre, Al-Amodi Autism Research Chair,
King Abdul Aziz City for Science and Technology (KACST), National Plan for
Science and Technology and Innovation (MAARIFAH), and Vice Deanship of Research
Chairs, at King Saud University, Kingdom of Saudi Arabia, for financial support.
The authors also thank the Department of Pharmacology, Faculty of Medicine, for
hosting the Autism Research and Treatment Centre Laboratory.
References
1. American
Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders,
Text Revision (DSM-IV-TR). Washington: American Psychiatric Association; 2000.
2. Kohane IS,
McMurry A, Weber G, et al. The co-morbidity burden of children and young adults
with autism spectrum disorders. PLoS One. 2012;7:e33224. [PMC
free article] [PubMed]
3. Bjorklund G,
Saad K, Chirumbolo S, et al. Immune dysfunction and neuroinflammation in autism
spectrum disorder. Acta Neurobiol Exp (Wars) 2016;76:257268. [PubMed]
4. El-Ansary A,
Hassan WM, Qasem H, et al. Identification of biomarkers of impaired sensory
profiles among autistic patients. PLoS One. 2016;11:e0164153. [PMC
free article] [PubMed]
5. Perry A,
Condillac RA, Freeman NL, et al. Multi-site study of the Childhood Autism Rating
Scale (CARS) in five clinical groups of young children. J Autism Dev Disord.
2005;35:625634. [PubMed]
6. Constantino JN,
Gruber CP. Social Responsiveness Scale (SRS) Los Angeles: Western Psychological
Services; 2007.
7. Dunn W. The
Sensory Profile: Examiner's Manual. San Antonio: Psychological Corporation;
1999.
8. Sokhadze EM,
Casanova MF, Tasman A, et al. Electrophysiological and behavioral outcomes of
Berard Auditory Integration Training (AIT) in children with autism spectrum
disorder. Appl Psychophysiol Biofeedback. 2016;41:405420. [PubMed]
9. Rogers S,
Vismara L. Evidence-based comprehensive treatments for early autism. J Clin
Child Adolesc Psychol. 2008;37:838. [PMC
free article] [PubMed]
10. Paul R.
Interventions to improve communication in autism. Child Adolesc Psychiatr Clin N
Am. 2008;17:835856. [PMC
free article] [PubMed]
11.
Tager-Flusberg H, Kasari C. Minimally verbal school-aged children with autism
spectrum disorder: the neglected end of the spectrum. Autism Res.
2013;6:468478. [PMC
free article] [PubMed]
12. Sinha Y,
Silove N, Hayen A, et al. Auditory integration training and other sound
therapies for autism spectrum disorders (ASD) Cochrane Database Syst Rev.
2011;7:12. [PubMed]
13. Berard G.
Hearing Equals Behavior. New Canaan: Keats; 1993.
14. Zhang GQ,
Gong Q, Zhang FL, et al. Effects of auditory integrative training on autistic
children. Beijing Da Xue Xue Bao. 2009;41:426431. [PubMed]
15. Russo NM,
Hornickel J, Nicol T, et al. Biological changes in auditory function following
training in children with autism spectrum disorders. Behav Brain Funct.
2010;6:60. [PMC
free article] [PubMed]
16. Aihara K,
Ikeda Y, Yagi S, et al. Transforming growth factor-β1 as a common target
molecule for development of cardiovascular diseases, renal insufficiency and
metabolic syndrome. Cardiol Res Pract. 2011;2011:175381. [PMC
free article] [PubMed]
17. Khakzad MR,
Salari F, Javanbakht M, et al. Transforming growth factor beta 1 869T/C and
915G/C polymorphisms and risk of autism spectrum disorders. Rep Biochem Mol
Biol. 2015;3:8288. [PMC
free article] [PubMed]
18. Kulkarni AB,
Huh CG, Becker D, et al. Transforming growth factor beta 1 null mutation in mice
causes excessive inflammatory response and early death. Proc Natl Acad Sci USA.
1993;90:770774. [PMC
free article] [PubMed]
19. Wyss-Coray T,
Borrow P, Brooker MJ, et al. Astroglial overproduction of TGF-beta 1 enhances
inflammatory central nervous system disease in transgenic mice. J Neuroimmunol.
1997;77:4550. [PubMed]
20. Prehn JH,
Backhauss C, Krieglstein J. Transforming growth factor-beta 1 prevents glutamate
neurotoxicity in rat neocortical cultures and protects mouse neocortex from
ischemic injury in vivo. J Cereb Blood Flow Metab. 1993;13:521525. [PubMed]
21. Vargas DL,
Nascimbene C, Krishnan C, et al. Neuroglial activation and neuroinflammation in
the brain of patients with autism. Ann Neurol. 2005;57:6781. [PubMed]
22. Okada K,
Hashimoto K, Iwata Y, et al. Decreased serum levels of transforming growth
factor-beta1 in patients with autism. Prog Neuropsychopharmacol Biol Psychiatry.
2007;31:187190. [PubMed]
23. Hashim H,
Abdelrahman H, Mohammed D, et al. Association between plasma levels of
transforming growth factor-b1, IL-23 and IL-17 and the severity of autism in
Egyptian children. Res Autism Spect Dis. 2013;7:199204.
24. Ashwood P,
Enstrom A, Krakowiak P, et al. Decreased transforming growth factor beta1 in
autism: a potential link between immune dysregulation and impairment in clinical
behavioral outcomes. J Neuroimmunol. 2008;204:149153. [PMC
free article] [PubMed]
25. Bashir S,
Zeina R, Muhammad D, et al. Role of hedgehog protein family members in autistic
children. Neurol Psychiatry Brain Res. 2014;20:6367.
26. Constantino
JN, Davis SA, Todd RD, et al. Validation of a brief quantitative measure of
autistic traits: comparison of the Social Responsiveness Scale with the autism
diagnostic interview-revised. J Autism Dev Disord. 2003;33:427433. [PubMed]
27. Al-Ayadhi LY,
Al-Drees AM, Al-Arfaj AM. Effectiveness of auditory integration therapy in
autism spectrum disorders - prospective study. Autism Insights. 2013;5:1320.
28. Sinha Y,
Silove N, Wheeler D, et al. Auditory integration training and other sound
therapies for autism spectrum disorders: a systematic review. Arch Dis Child.
2006;91:10181022. [PMC
free article] [PubMed]
29. Hall L,
Case-Smith J. The effect of sound-based intervention on children with sensory
processing disorders and visual-motor delays. Am J Occup Ther. 2007;61:209215.
[PubMed]
30. Pfeiffer BA,
Koenig K, Kinnealey M, et al. Effectiveness of sensory integration interventions
in children with autism spectrum disorders: a pilot study. Am J Occup Ther.
2011;65:7685. [PMC
free article] [PubMed]
31. Depino AM,
Lucchina L, Pitossi F. Early and adult hippocampal TGF-β1 overexpression have
opposite effects on behavior. Brain Behav Immun. 2011;25:15821591. [PubMed]
32. Coccaro EF,
Lee R, Coussons-Read M. Cerebrospinal fluid inflammatory cytokines and
aggression in personality disordered subjects. Int J Neuropsychopharmacol.
2015;18:pyv001. [PMC
free article] [PubMed]
33. Fiorentino M,
Sapone A, Senger S. Blood-brain barrier and intestinal epithelial barrier
alterations in autism spectrum disorders. Mol Autism. 2016;7:49. [PMC
free article] [PubMed]
| 






